|
 |
|
Recent Publications Lixisenatide, a novel GLP-1 receptor agonist: efficacy, safety and clinical implications for type 2 diabetes mellitus. GB Bolli, DR Owens, Diabetes Obes. Metab. , 2014, 16, 588-601. doi: 10.1111/dom.12253 Recent advances in therapies for the treatment of type 2 diabetes mellitus (T2DM) have led to the development of glucagon-like peptide-1 receptor agonists (GLP-1 RAs), which, unlike insulin and sulphonylurea, are effective, with a low risk of hypoglycaemia. Lixisenatide is recommended as a once-daily GLP-1 RA for the treatment of T2DM. In persons with T2DM, lixisenatide 20µg once-daily given by bolus subcutaneous injection improves insulin secretion and suppresses glucagon secretion in a glucose-dependent manner. Compared with the longer-acting GLP-1 RA liraglutide, lixisenatide achieved a significantly greater reduction in postprandial plasma glucose (PPG) during a standardized test breakfast in persons with T2DM otherwise insufficiently controlled on metformin alone. This is primarily due to the greater inhibition of gastric motility by lixisenatide compared with liraglutide. The efficacy and safety of lixisenatide was evaluated across a spectrum of T2DM in a series of phase III, randomized, placebo-controlled trials known as the GetGoal programme. Lixisenatide monotherapy or as add-on to oral antidiabetic agents or basal insulin achieved significant reductions in glycated haemoglobin, PPG and fasting plasma glucose, with either weight loss or no weight gain. The most frequent adverse events were gastrointestinal and transient in nature. Lixisenatide provides an easy, once-daily, single-dose, add-on treatment to oral antidiabetic agents or basal insulin for the management of T2DM, with little or no increased risk of hypoglycaemia and a potential beneficial effect on body weight.
Site-Selective Solid-Phase Synthesis of a CCR5 Sulfopeptide Library to Interrogate HIV Binding and Entry X Liu, LR Malins, M Roche,J Sterjovski, R Duncan, ML Garcia, NC Barnes, DA Anderson, MJ Stone, PR Gorry, RJ Payne, ACS Chem. Biol., Just Accepted Manuscript DOI: 10.1021/cb500337r Publication Date (Web): June 25, 2014 Copyright © 2014 American Chemical Society Tyrosine (Tyr) sulfation is a common post-translational modification that is implicated in a variety of important biological processes, including the fusion and entry of human immunodeficiency virus type-1 (HIV-1). A number of sulfated Tyr (sTyr) residues on the N-terminus of the CCR5 chemokine receptor are involved in a crucial binding interaction with the gp120 HIV-1 envelope glycoprotein. Despite the established importance of these sTyr residues, the exact structural and functional role of this post-translational modification in HIV-1 infection is not fully understood. Detailed biological studies are hindered in part by the difficulty in accessing homogeneous sulfopeptides and sulfoproteins through biological expression and established synthetic techniques. Herein we describe an efficient approach to the synthesis of sulfopeptides bearing discrete sulfation patterns through the divergent, site-selective incorporation of sTyr residues on solid support. By employing three orthogonally protected Tyr building blocks and a solid-phase sulfation protocol, we demonstrate the synthesis of a library of target N-terminal CCR5(2-22) sulfoforms bearing discrete and differential sulfation at Tyr10, Tyr14 and Tyr15, from a single resin-bound intermediate. We demonstrate the importance of distinct sites of Tyr sulfation in binding gp120 through a competitive binding assay between the synthetic CCR5 sulfopeptides and an anti-gp120 monoclonal antibody. These studies revealed a critical role of sulfation at Tyr14 for binding and a possible additional role for sulfation at Tyr10. N-terminal CCR5 variants bearing a sTyr residue at position 14 were also found to complement viral entry into cells expressing an N-terminally truncated CCR5 receptor. Inhibition of agronomically relevant fungal phytopathogens by tyrocidines, cyclic antimicrobial peptides isolated from Bacillus aneurinolyticus. AM Troskie, A de Beer, JA Vosloo, K Jacobs, M Rautenbach, Microbiology, 2014, Jul 4. doi: 10.1099/mic.0.078840-0. [Epub ahead of print] The tyrocidines, a complex of analogous cyclic decapeptides produced by Bacillus aneurinolyticus, exhibited noteworthy activity against a range of phytopathogenic fungi, including Fusarium verticillioides, F. solani and Botrytis cinerea. The activity of the tyrocidine peptide complex (Trc mixture) and purified tyrocidines exhibited minimum inhibition concentrations below 13 μg/mL (≠10 μM) and was significantly more potent than that of the commercial imidazole fungicide, bifonazole. Although the tyrocidines' activity was negatively influenced by the presence of Ca2+, it remained unaffected by the presence of Mg2+, Na+ and K2+. Microscopic analysis revealed significant impact on the morphology of F. solani and B. cinerea including retarded germination and hyperbranching of hyphae. Studies with membrane impermeable dyes, SYTOX green and propidium iodide, suggest that the tyrocidine main mode of action involves the disruption of fungal membrane integrity. Because of the tyrocidines' broad spectrum and potent antifungal activity, possible multiple targets reducing the risk of overt resistance and general salt tolerance, they are promising candidates that warrant further investigation as bio-fungicides. Copyright © 2014, the Society for General Microbiology. The Novel GLP-1-Gastrin Dual Agonist ZP3022 Improves Glucose Homeostasis and Increases β-Cell Mass without Affecting Islet Number in db/db Mice. LS Dalboge, DL Almholt, TS Neerup, N Vrang, J Jelsing, K Fosgerau, J. Pharmacol. Exp. Ther., 2014, 350, 353-60. doi: 10.1124/jpet.114.215293. Epub 2014 Jun 5. Antidiabetic treatments aiming to preserve or even to increase β-cell mass are currently gaining increased interest. Here we investigated the effect of chronic treatment with the novel glucagon-like peptide-1 (GLP-1)-gastrin dual agonist ZP3022 (HGEGTFTSDLSKQMEEEAVRLFIE-WLKN-8Ado-8Ado-YGWLDF-NH2, 8-Ado= 8-amino-3,6-dioxo-octanoic acid) on glycemic control, β-cell mass and proliferation, and islet number. Male db/db mice were treated with ZP3022, liraglutide, or vehicle for 2, 4, or 8 weeks, with terminal assessment of hemoglobin A1c, basal blood glucose, and plasma insulin concentrations. Pancreata were removed for immunohistochemical staining and stereological quantification of β-cell mass, islet numbers, proliferation, and apoptosis. Treatment with ZP3022 or liraglutide led to a significant improvement in glycemic control. ZP3022 treatment resulted in a sustained increase in β-cell mass after 4 and 8 weeks of treatment, whereas the effect of liraglutide was transient. The expansion in β-cell mass observed in the ZP3022-treated mice appeared to be driven by increased β-cell proliferation in existing islets rather than by formation of new islets, as mean islet mass increased but the number of islets remained constant. Our data demonstrate that the GLP-1-gastrin dual agonist ZP3022 causes a sustained improvement in glycemic control accompanied by an increase in β-cell mass, increased proliferation, and increased mean islet mass. The results highlight that the GLP-1-gastrin dualagonist increases β-cell mass more than liraglutide and that dual agonists could potentially be developed into a new class of antidiabetic treatments. Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
|
|
Special Summer Savings AAPPTec is offering a special summer discount on 250g packages of common Fmoc-amino acids. Through August and September, AAPPTec is offering 250g packages of the common amino acids at significant discounts. Place your order online for 3 or more 250g packages between August 1 and September 15, and use the special code “Summer Savings” to obtain these special prices. These special discounted prices are only for 250g packages. Standard catalog prices apply for all quantities below 250g.
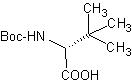
These prices are only valid for orders placed by September 15. This offer may not be used in combination with other offers or discounts. α-Methyl Phenylalanines 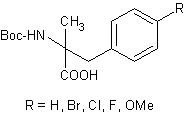 AAPPTec now offers both D and L isomers of Boc-α-Me-Phe-OH and ring substituted derivates.
Recently Added Products
Apex 396 HT Parallel Peptide Library Synthesizer
The Apex 396HT is AAPPTec’s latest high throughput parallel peptide synthesizer that can prepare 192 different peptides at the same time in titer plates. The Apex 396HT can prepare up to 96 peptides in larger quantities utilizing standard AAPPTec reactor assemblies. The Apex 396HT features two racks each holding 36 amino acid vessels and four 800 mL reagent bottles. For more information about the new Apex 396HT, please send an email to info@aapptec.com. |
 |
UPCOMING EVENTS |
|
|
August 2014 ACS Fall August 10-14 AFEBS-EMBO Joint Conference August 30-September 4 European Peptide Symposium August 31-September 5 |
 |