|
 |
|
Recent Publications Biological and Structural Characterization of a Naturally Inspired Material Engineered from Elastin as a Candidate for Tissue Engineering Applications M Vassalli, F Sbrana, A Laurita, M Papi, N Bloise, L Visai, B Bochicchio Langmuir, Article ASAP DOI: 10.1021/la403311x Publication Date (Web): December 5, 2013 Copyright © 2013 American Chemical Society The adoption of a biomimetic approach in the design and fabrication of innovative materials for biomedical applications is encountering a growing interest. In particular, new molecules are being engineered on the basis of proteins present in the extracellular matrix, such as fibronectin, collagen, or elastin. Following this approach scientists expect to be able not only to obtain materials with tailored mechanical properties but also to elicit specific biological responses inherited by the mimicked tissue. In the present work, a novel peptide, engineered starting from the sequence encoded by exon 28 of human tropoelastin, was characterized from a chemical, physical, and biological point of view. The obtained molecule was observed to aggregate at high temperatures, forming a material able to induce a biological effect similar to what elastin does in the physiological context. This material seems to be a good candidate to play a relevant role in future biomedical applications with special reference to vascular surgery. Inhibiting and Reversing Amyloid-β Peptide (1-40) Fibril Formation with Gramicidin S and Engineered Analogues. J Luo, JM Otero, CH Yu, SK Warmlander, A Graslund, M Overhand, JP Abrahams., Chemistry, 2013, 19, 17338-48. doi: 10.1002/chem.201301535. In Alzheimer's disease, amyloid-β (Aβ) peptides aggregate into extracellular fibrillar deposits. Although these deposits may not be the prime cause of the neurodegeneration that characterizes this disease, inhibition or dissolution of amyloid fibril formation by Aβ peptides is likely to affect its development. ThT fluorescence measurements and AFM images showed that the natural antibiotic gramicidin S significantly inhibited Aβ amyloid formation in vitro and could dissolve amyloids that had formed in the absence of the antibiotic. In silico docking suggested that gramicidin S, a cyclic decapeptide that adopts a β-sheet conformation, binds to the Aβ peptide hairpin-stacked fibril through β-sheet interactions. This may explain why gramicidin S reduces fibril formation. Analogues of gramicidin S were also tested. An analogue with a potency that was four-times higher than that of the natural product was identified. Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Truncated and constrained helical analogs of antimicrobial esculentin-2EM TK Pham, DH Kim, BJ Lee, YW Kim., Bioorg Med Chem Lett., 2013, 23, 6717-20. doi: 10.1016/j.bmcl.2013.10.031. Esculentin-2EM is a 37-residue, cationic, amphipathic, α-helical antimicrobial peptide isolated from a Korean frog, Glandirama emeljanovi. Many studies revealed that truncation of this peptide results in substantial decreases in its antimicrobial activity. Lee and his colleagues have recently reported that a 23-residue esculentin-2EM analog containing a tryptophanyl substitution at position 16 showed a significant recovery of the antimicrobial activity of the parent peptide. Here we report a new series of 15-residue esculentin-2EM analogs which are constrained into an α-helical conformation via an oct-4-enyl cross-link. The resulting 'stapled' derivatives displayed remarkable increases not only in antimicrobial activity but also in helical content and protease resistance compared to Lee's original 23-residue esculentin-2EM analog. The preliminary data obtained in this work strongly supports the potential of our strategy for the development of a new class of peptide antibiotics. Copyright © 2013 Elsevier Ltd. All rights reserved. Kisspeptin is providing new insights into the control of reproduction C Izzi-Engbeaya, K Meeran, WS Dhillo, Clin. Med., 2013, 13, 547-8. doi: 10.7861/clinmedicine.13-6-547. The kisspeptins are a group of recently discovered peptide hormones (collectively termed kisspeptin), which play a pivotal role in reproduction. Research investigating the actions of kisspeptin is helping to elucidate the regulatory mechanisms which govern fertility and may lead to the development of novel treatments for some reproductive disorders. A bumblebee (Bombus ignitus) venom serine protease inhibitor that acts as a microbial serine protease inhibitor H Wan, BY Kim, KS Lee, HJ Yoon, KY Lee, BR Jin, Comp Biochem Physiol B Biochem Mol Biol. 2014, 167, 59-64. doi: 10.1016/j.cbpb.2013.10.002. Serine protease inhibitors from bumblebee venom have been shown to block plasmin activity. In this study, we identified the protein BiVSPI from the venom of Bombus ignitus to be a serine protease inhibitor and an antimicrobial factor. BiVSPI is a 55-amino acid mature peptide with ten conserved cysteine residues and a P1 methionine residue. BiVSPI is expressed in the venom gland and also found in the venom as an 8-kDa peptide. Recombinant BiVSPI that was expressed in baculovirus-infected insect cells exhibited inhibitory activity against chymotrypsin but not trypsin. BiVSPI also inhibited microbial serine proteases, such as subtilisin A (Ki=6.57nM) and proteinase K (Ki=7.11nM). In addition, BiVSPI was shown to bind directly to Bacillus subtilis, Bacillus thuringiensis, and Beauveria bassiana but not to Escherichia coli. Consistent with these results, BiVSPI exhibited antimicrobial activity against Gram-positive bacteria and fungi. These findings provide evidence for a novel serine protease inhibitor in bumblebee venom that has antimicrobial functions. © 2013 Elsevier Inc. All rights reserved. Evaluation of acid-labile S-protecting groups to prevent Cys racemization in Fmoc solid-phase peptide synthesis H Hibino, Y Miki, Y Nishiuchi, J. Pept. Sci., Early View (Online Version of Record published before inclusion in an issue) Article first published online: 28 NOV 2013 DOI: 10.1002/psc.2585 Phosphonium and uronium salt-based reagents enable efficient and effective coupling reactions and are indispensable in peptide chemistry, especially in machine-assisted SPPS. However, after the activating and coupling steps with these reagents in the presence of tertiary amines, Fmoc derivatives of Cys are known to be considerably racemized during their incorporation. To avoid this side reaction, a coupling method mediated by phosphonium/uronium reagents with a weaker base, such as 2,4,6-trimethylpyridine, than the ordinarily used DIEA or that by carbodiimide has been recommended. However, these methods are appreciably inferior to the standard protocol applied for SPPS, that is, a 1min preactivation procedure of coupling with phosphonium or uronium reagents/DIEA in DMF, in terms of coupling efficiency, and also the former method cannot reduce racemization of Cys(Trt) to an acceptable level (<1.0%) even when the preactivation procedure is omitted. Here, the 4,4'-dimethoxydiphenylmethyl and 4-methoxybenzyloxymethyl groups were demonstrated to be acid-labile S-protecting groups that can suppress racemization of Cys to an acceptable level (<1.0%) when the respective Fmoc derivatives are incorporated via the standard SPPS protocol of phosphonium or uronium reagents with the aid of DIEA in DMF. Furthermore, these protecting groups significantly reduced the rate of racemization compared to the Trt group even in the case of microwave-assisted SPPS performed at a high temperature. © 2013 The Authors. European Peptide Society published by John Wiley & Sons, Ltd. BOP-OXy, BOP-OBt, and BOP-OAt: novel organophosphinic coupling reagents useful for solution and solid-phase peptide synthesis A El-Faham, SN Khattab, R Subiros-Funosas, F Albericio, J. Pept. Sci., Early View (Online Version of Record published before inclusion in an issue) Article first published online: 22 NOV 2013 DOI: 10.1002/psc.2579 Stand-alone coupling reagents derived from bis(2-oxo-3-oxazolidinyl)phosphorodiamidic chloride show efficient performance in solution and SPPS. In particular, the Oxyma Pure (Luxembourg Biotech., Tel Aviv, Israel) derivative shows the additional advantage of being highly soluble in DMF and even fairly soluble in CH3CN, which can extend its use for the synthesis of complex peptides. These new stand-alone coupling reagents have the advantage of not bearing any counteranion such as PF6 or BH4, whose presence can jeopardize the purification of final peptides prepared in solution. Copyright © 2013 European Peptide Society and John Wiley & Sons, Ltd. Replacement of the Disulfide Bridge in a KLK3-Stimulating Peptide Using Orthogonally Protected Building Blocks K Meinander, M Pakkala, J Weisell, U-H Stenman, H Koistinen, A Narvanen, EAA Wallen ACS Med. Chem. Lett., Article ASAP DOI: 10.1021/ml400419g Publication Date (Web): December 16, 2013 Copyright © 2013 American Chemical Society Peptide "B-2", which is one of the most potent kallikrein-related peptidase 3 (KLK3)-stimulating compounds, consists of 12 amino acids and is cyclized by a disulfide bridge between the N- and C-terminal cysteines. Orthogonally protected building blocks were used in the peptide synthesis to introduce a disulfide bridge mimetic consisting of four carbon atoms. The resulting pseudopeptides with alkane and E-alkene linkers doubled the proteolytic activity of KLK3 at a concentration of 14 μM. They were almost as potent as the parent "B-2" peptide, which gives a 3.6-fold increase in the proteolytic activity of KLK3 at the same concentration. An apolipoprotein E mimetic peptide with activities against multidrug-resistant bacteria and immunomodulatory effects. CQ Wang, CS Yang, Y Yang, F Pan, LY He, AM Wang, J. Pept. Sci., 2013, 19, 745-50. doi: 10.1002/psc.2570. Apolipoprotein E (apoE) mimetic peptides derived from the low-density lipoprotein receptor-binding region of apoE with both activities against multidrug-resistant bacteria and immunomodulatory effects have not previously been reported. We identified an apoE mimetic peptide analogue of the receptor-binding region of apoE (abbreviated as apoE23) with the sequence of LRKLRKRLVRLASHLRKLRKRLL, which exhibited high antibacterial effects. The minimal inhibitory concentration of apoE23 against multidrug-resistant Acinetobacter baumannii was 6 μg/ml. The antimicrobial activity of apoE23 depended on its amphipathic α-helical conformation. Moreover, apoE23 downregulated the expression of tumour necrosis factor-α, interleukin-6 and interleukin-10 in lipopolysaccharide-induced THP-1 cells. ApoE23 exhibits potential in future clinical applications. Copyright © 2013 European Peptide Society and John Wiley & Sons, Ltd. Scorpion toxins for the reversal of BoNT-induced paralysis CA Lowery, M Adler, A Borrell, KD Janda, Bioorg. Med. Chem. Lett., 2013, 23, 6743-6. doi: 10.1016/j.bmcl.2013.10.029. The botulinum neurotoxins, characterized by their neuromuscular paralytic effects, are the most toxic proteins known to man. Due to their extreme potency, ease of production, and duration of activity, the BoNT proteins have been classified by the Centers for Disease Control as high threat agents for bioterrorism. In an attempt to discover effective BoNT therapeutics, we have pursued a strategy in which we leverage the blockade of K(+) channels that ultimately results in the reversal of neuromuscular paralysis. Towards this end, we utilized peptides derived from scorpion venom that are highly potent K(+) channel blockers. Herein, we report the synthesis of charybdotoxin, a 37 amino acid peptide, and detail its activity, along with iberiotoxin and margatoxin, in a mouse phrenic nerve hemidiaphragm assay in the absence and the presence of BoNT/A. Copyright © 2013 Elsevier Ltd. All rights reserved. Activation of urocortin 1 and ghrelin signaling in the basolateral amygdala induces anxiogenesis PJ Currie, LM Schuette, SE Wauson, WN Voss, MJ Angeles, Neuroreport., 2014, 25, 60-4. doi: 10.1097/WNR.0000000000000047. Prior anatomical and functional studies have demonstrated the importance of the basolateral region of the amygdala in the regulation of anxiogenic and anxiolytic behaviors. In the present report we investigated the anxiety-inducing effects of the corticotropin-releasing hormone-related peptide urocortin 1 (Ucn1) and the gut-brain peptide ghrelin. Both peptides were injected directly into the basolateral amygdala of male Sprague-Dawley rats and performance in the elevated plus maze was assessed. Ghrelin was administered at doses of 3-300 pmol and Ucn1 at doses of 0.01-1.0 pmol. Separate groups of rats were pretreated with Ucn1 before ghrelin treatment. In all experiments each test was performed as a single trial per animal. Results indicated that both ghrelin and Ucn1 elicited an increase in anxiogenic behavior. Moreover, Ucn1 pretreament potentiated the anxiogenic action of ghrelin. Overall these findings provide support for an integrated role of ghrelin and urocortin signaling within the basolateral amygdala in the expression of anxiogenesis. A Lys-Trp cation-Π interaction mediates dimerisation and function of the chloride intracellular channel protein 1 transmembrane domain B Peter, AA Polyansky, S Fanucchi, HW Dirr, Biochemistry, Just Accepted Manuscript DOI: 10.1021/bi401433f Publication Date (Web): December 11, 2013 Copyright © 2013 American Chemical Society The chloride intracellular channel protein 1 (CLIC1) is a dual-state protein that can exist either as a soluble monomer or in an integral membrane form. The oligomerisation of the transmembrane domain (TMD) remains speculative despite being implicated in pore formation. The extent to which electrostatic and van der Waals interactions drive folding and association of the dimorphic TMD is unknown and is complicated by the requirement of interactions favourable in both aqueous and membrane environments. Here we report a putative Lys37-Trp35 cation-π interaction and show that it stabilises the dimeric form of the CLIC1 TMD in membranes. A synthetic 30-mer peptide comprising a K37M TMD mutant was examined in 2,2,2-trifluoroethanol (TFE), SDS micelles and POPC liposomes using far-UV CD, fluorescence and UV absorbance spectroscopy. Our data suggest that Lys37 is not implicated in the folding, stability or membrane insertion of the TMD peptide. However, removal of this residue severely impairs the formation of dimers and higher order oligomers. This is accompanied by a 35-fold loss in chloride influx activity, suggesting that dimerisation modulates the rate of chloride conductance. We propose that, within membranes, individual TMD helices associate via a Lys37-mediated cation-π interaction to form active dimers. The latter findings are also supported by results of modelling a putative TMD dimer conformation where Lys37 and Trp35 form cation-π pairs at the dimer interface. Dimeric helix bundles may then associate to form fully-active ion-channels. Thus, within a membrane-like environment, aromatic interactions involving a polar lysine side chain provide a thermodynamic driving force for helix-helix association. |
|
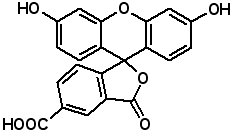
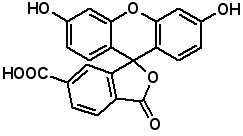
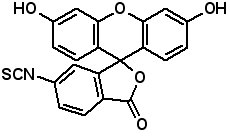
Fluorescent Dyes Fluorescence labeled peptides are used to study enzyme kinetics, transport of peptides into cells and the disposition of peptides within cells, tissues and organs. AAPPTec offers five fluorescein-based reagents for preparing labeled peptides, 5-FAM, 6-FAM, 5(6)-FAM, 5-FITC and 6-FITC. These reagents react with amines to form labeled compounds that fluoresce green when illuminated with UV light.
Focus XC The Focus XC is the ideal research and production peptide synthesizer. It can synthesize up to six different peptides at the
same time in scales from milligrams to grams. The Focus XC is easy to operate. The easy to use software allows novices to prepare high quality peptides, but also offers flexibility for
experts to develop new protocols and optimize the synthesis of difficult peptides.
Azidoamino acids undergo Cu(I) catalyzed cycloaddition reactions with alkynes to form 1,2,3 triazoles. This reaction proceeds in high yield, is compatible with a wide range of solvents including water, and no byproducts are formed. This has become a popular method for conjugating peptides to dyes, labels or proteins. (S Maschauer, R Haubner, T Kuwert, OPrante,
Mol. Pharmaceutics, Articles ASAP (As Soon As Publishable) Publication Date (Web): December 10, 2013; T Liu, Z Qian, Q Xiao, D Pei, ACS Comb. Sci., 2011, 13, 537-546; A Krause, A Kirschning, G. Drager, Org. Biomol. Chem., 2012, 10, 5547-53.) It has also been utilized to form cyclic or stapled peptides. (JH Park, ML Waters, Org. Biomol. Chem., 2013, 7, 11, 69-77; M Scrima, M Grimaldi, S Di Marino, et al., Biopolymers., 2012, 98, 535-45; Jacobsen, H Maekawa, N-H Ge, et al., J. Org. Chem., 2011, 76, 1228-1238; T Iwata, K Tanaka, T Tahara, et al., Mol. Biosyst., 2013, 9, 1019-25.)
MSDS Available Online Material Safety Data Sheets (MSDS) for all AAPPTec chemical products are available on-line through AAPPTec's convenient on-line catalog. Click on the product in the catalog, then click on the MSDS link to download the MSDS. Apex 396
The Apex 396 is the premier productivity tool. Capable of preparing a few peptides or up to 96 separate peptides at the same time, the Apex 396 is ideal for SAR studies, optimization studies, drug discovery, and small scale peptide production.
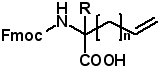
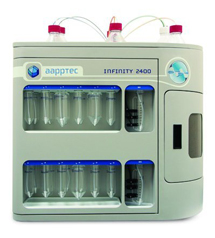
 The Apex 396HT can prepare up to 96 peptides in larger quantities utilizing standard AAPPTec reactor assemblies. The Apex 396HT features four 800 mL reagent bottles and two racks each holding 36 amino acid vessels. For more information about the Apex 396HT, please visit the Apex 396 HT page at www.aapptec.com. Alkenyl Amino Acids for Preparing Stapled Peptides R=H, Me AAPPTec provides the largest selection of alkenyl amino acids, the building blocks used to prepare stapled peptides. Stapled peptides have greater helical structure which may impart improved drug properties such as greater activity, improved transport through cell membranes, and greater resistance to protease enzymes. To view AAPPTec's selection of alkenyl amino acids, CLICK HERE. Infinity 2400TM Microwave Peptide Synthesizer
Slow or difficult deprotection and coupling reactions reduce yields and result in crude peptides containing truncation and deletion peptide impurities. AAPPTec's Infinity 2400 Peptide Synthesizer utilizes microwaves for rapid heating that can accelerate slow reactions, improve yields and produce purer peptides of difficult sequences.
Apex 396 Methylation of lysine residue side-chains plays a regulatory role in histones and other proteins. Study of these regulatory processes and the enzymes involved requires suitable substrates, analogs and model peptides. AAPPTec supplies modified lysine derivatives suitable for preparing peptide substrate analogs of these regulatory sites in either research or bulk quantities. Please send an e-mail to sales@aapptec.com for a quotation on larger quantities. .jpg)
2 HCl.jpg)
|
 |
UPCOMING EVENTS |
|
|
February 2014 Chemistry and Biology of Peptides, Gordon Conference February 23-28 5th Asian TIDES February 25-27 |
 |

3Cl.jpg)