|
 |
|
Featured Articles Total chemical synthesis of fully functional Photoactive Yellow Protein. Gordon WR, Bang D, Hoff WD, Kent SB. Bioorg Med Chem. 2013 Jun 15;21(12):3436-42. doi: 10.1016/j.bmc.2013.03.025. Epub 2013 Mar 27. The Photoactive Yellow Protein (PYP) is a structural prototype for the PAS superfamily of proteins, which includes hundreds of receptor and regulatory proteins from all three kingdoms of life. PYP itself is a small globular protein that undergoes a photocycle involving a series of conformational changes in response to light excitation of its p-coumaric acid chromophore, making it an excellent model system to study the molecular basis of signaling in the PAS super family. To enable novel chemical approaches to elucidating the structural changes that accompany signaling in PYP, we have chemically synthesized the 125 amino acid residue protein molecule using a combination of Boc chemistry solid phase peptide synthesis and native chemical ligation. Synthetic PYP exhibits the wildtype photocycle, as determined in photobleaching studies. Planned future studies include incorporation of site-specific isotopic labels into specific secondary structural elements to determine which structural elements are involved in signaling state formation using difference FTIR spectroscopy. Copyright © 2013. Published by Elsevier Ltd. Identification of a Hepatoprotective Peptide in Wheat Gluten Hydrolysate against D-Galactosamine-Induced Acute Hepatitis in Rat Kenji Sato , Yukari Egashira , Shin Ono , Satoshi Mochizuki , Yuki Shimmura , Yoshio Suzuki , Megumi Nagata , Kaori Hashimoto , Tamami Kiyono , Eun Young Park , Yasushi Nakamura , Mariko Itabashi , Yuka Sakata , Seigo Furuta , and Hiroo Sanada J. Agric. Food Chem., Just Accepted Manuscript DOI: 10.1021/jf400914e Publication Date (Web): June 7, 2013 Copyright © 2013 American Chemical Society A hepatoprotective peptide, pyroglutamyl leucine (pyroGlu-Leu), was identified in wheat gluten hydrolysate through an in vivo activity-guided fractionation approach based on D-galactosamine-induced acute hepatitis in rat and fractionation of peptides with large-scale preparative ampholine-free isoelectric focusing. The active acidic fraction predominantly consisted of pyroglutamyl peptides and free pyroglutamic acid. Pyroglutamyl peptides were derivatized with phenyl isothiocyanate after removal of pyroglutamyl residue by pyroglutamate aminopeptidase. The derivatives were purified by reversed-phase HPLC and subjected to sequence analysis. The active fraction contained pyroGlu-Ile, pyroGlu-Leu, pyroGlu-Gln, pyroGlu-Gln-Gln, and free pyroGlu. Ingestion of pyroGlu-Leu at 20 mg/kg body weight significantly decreased serum aspartate and alanine aminotransferases to approximately 30 and 20% of those values of vehicle group, respectively, which were near the normal levels. Thirty min after ingestion of pyroGlu-Leu at 20 mg/kg, the concentration of pyroGlu-Leu in portal blood plasma increased to approximately 2µM. Dicarba a-Conotoxin Vc1.1 Analogues with Differential Selectivity for Nicotinic Acetylcholine and GABAB Receptors Bianca J. van Lierop, Samuel D. Robinson, Shiva N. Kompella, Alessia Belgi, Jeffrey R. McArthur, Andrew Hung, Christopher A. MacRaild, David J. Adams, Raymond S. Norton, and Andrea J. Robinson ACS Chem. Biol., Article ASAP DOI: 10.1021/cb4002393 Publication Date (Web): June 2, 2013 Copyright © 2013 American Chemical Society Conotoxins have emerged as useful leads for the development of novel therapeutic analgesics. These peptides, isolated from marine molluscs of the genus Conus, have evolved exquisite selectivity for receptors and ion channels of excitable tissue. One such peptide, a-conotoxin Vc1.1, is a 16-mer possessing an interlocked disulfide framework. Despite its emergence as a potent analgesic lead, the molecular target and mechanism of action of Vc1.1 have not been elucidated to date. In this paper we describe the regioselective synthesis of dicarba analogues of Vc1.1 using olefin metathesis. The ability of these peptides to inhibit acetylcholine-evoked current at rat a9a10 and a3ß4 nicotinic acetylcholine receptors (nAChR) expressed in Xenopus oocytes has been assessed in addition to their ability to inhibit high voltage-activated (HVA) calcium channel current in isolated rat DRG neurons. Their solution structures were determined by NMR spectroscopy. Significantly, we have found that regioselective replacement of the native cystine framework with a dicarba bridge can be used to selectively tune the cyclic peptide’s innate biological activity for one receptor over another. The 2,8-dicarba Vc1.1 isomer retains activity at γ-aminobutyric acid (GABAB) G protein-coupled receptors, whereas the isomeric 3,16-dicarba Vc1.1 peptide retains activity at the a9a10 nAChR subtype. These singularly acting analogues will enable the elucidation of the biological target responsible for the peptide’s potent analgesic activity. Design of Cell-Permeable Stapled-Peptides as HIV-1 Integrase Inhibitors Ya-Qiu Long , Shao-Xu Huang , Zahrah Zawahir , Zhong-Liang Xu , Hui-Yuan Li , Tino W. Sanchez , Ying Zhi , Stephanie De Houwer , Frauke Christ , Zeger Debyser , and Nouri Neamati J. Med. Chem., Just Accepted Manuscript DOI: 10.1021/jm4006516 Publication Date (Web): June 12, 2013 Copyright © 2013 American Chemical Society HIV-1 integrase (IN) catalyzes the integration of viral DNA into the host genome, involving several interactions with host proteins. We have previously identified peptide IN inhibitors derived from the alpha-helical regions along the dimeric interface of HIV-1 IN. Herein, we show that appropriate hydrocarbon-stapling of these peptides to stabilize their helical structure remarkably improves the cell permeability, thus allowing inhibition of the HIV-1 replication in cell culture. Furthermore, the stabilized peptides inhibit the interaction of IN with the cellular cofactor LEDGF/p75. Cellular uptake of the stapled peptide was confirmed in four different cell lines using a fluorescein-labeled analogue. Given their enhanced potency and cell permeability, these stapled peptides can serve as not only lead compounds of novel HIV-1 IN inhibitors but also prototypical biochemical probes or ‘nanoneedles’ for the elucidation of HIV-1 IN dimerization and host co-factor interactions within their native cellular environment. Surugamides A–E, Cyclic Octapeptides with Four d-Amino Acid Residues, from a Marine Streptomyces sp.: LC–MS-Aided Inspection of Partial Hydrolysates for the Distinction of d- and l-Amino Acid Residues in the Sequence Kentaro Takada, Akihiro Ninomiya, Masato Naruse, Yi Sun, Masayuki Miyazaki, Yuichi Nogi, Shigeru Okada, and Shigeki Matsunaga J. Org. Chem., Article ASAP DOI: 10.1021/jo400708u Publication Date (Web): June 7, 2013 Copyright © 2013 American Chemical Society Surugamides A–E (1–5), cyclic octapeptides with four d-amino acid residues, were isolated from the broth of marine-derived Streptomyces sp. Their planar structures were determined by analyses of spectroscopic data, and the absolute configuration of constituent amino acid residues was determined by the Marfey’s method. Differentiation of d-Ile and l-Ile in the sequence was established by chiral analysis of fragment peptides obtained from the partial hydrolysate, whose identification was conducted by LC–MS/MS. Chemical synthesis and orexigenic activity of rat/mouse relaxin-3. Hossain MA, Smith CM, Ryan PJ, Büchler E, Bathgate RA, Gundlach AL, Wade JD. Amino Acids. 2013 Jun;44(6):1529-36. doi: 10.1007/s00726-013-1478-0. Epub 2013 Mar 1. The insulin-like peptide, relaxin-3 was first identified just a decade ago via a genomic database search and is now recognized to be a key neuropeptide with several roles including the regulation of arousal, stress responses and neuroendocrine homeostasis. It also has significant potential as a drug to treat stress and obesity. Its actions are mediated via its cognate G protein-coupled receptor, RXFP3, which is found in abundant numbers in the brain. However, much remains to be determined with respect to the mechanism of neurological action of this peptide. Consequently, the chemical synthesis of the rat and mouse (which share identical primary structures) two-chain, three disulfide peptide was undertaken and the resulting peptide subjected to detailed in vitro and in vivo assay. Use of efficient solid-phase synthesis methods provided the two regioselectively S-protected A- and B-chains which were readily combined via sequential disulfide bond formation. The synthetic rat/mouse relaxin-3 was obtained in high purity and good overall yield. It demonstrated potent orexigenic activity in rats in that central intracerebroventricular infusion led to significantly increased food intake and water drinking. |
|
AAPPTec at APS in Hawaii The recent APS exhibition was very successful for AAPPTec. We debuted our newest instrument, the Infinity 2400™ Microwave Peptide Synthesizer. The Infinity 2400 can microwave up to six reactors at the same time. Reactor volumes of 5 mL to 100 mL are available, and single reactors up to 300 mL. Amino acids can be preactivated in a separate vessel at reduced temperature to minimize decomposition of the activated amino acids. .JPG) For more information about the Infinity 2400TM, visit our website at www.aapptec.com.
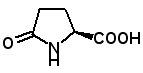
Pyroglutamic Acid Recently, the hepatoprotective dipeptide pyroglutamyl leucine was identified in wheat gluten hydrolysate (K. Sato, et al., J. Agric. Food Chem., Just Accepted Manuscript Published on Web June 7, 2013). AAPPTec offers pyroglutamic acid for preparing pyroglutamyl peptides.
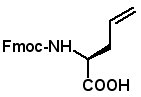
AllylGlycine
Dicarba analogs of disulfide bridges can be formed by replacing cysteine residues with allylglycine, then forming the bridge by ring closing metathesis. This strategy was recently applied to a-conotoxin Vc1.1. Regioselective replacement of the native disulfide bonds produced analogs selective for nicotinic acetylcholine receptors or γ-aminobutyric acid G protein-coupled receptors (B.J. van Lierop, et al. ACS Chem. Biol., Article ASAP Published on Web: June 2, 2013).
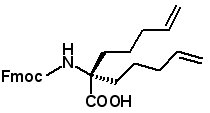
Stapled Peptide Building Blocks Hydrocarbon stapling to stabilize the a-helical structure of peptides can enhance peptide activity and cell permeability. Recently it was shown that stapling HIV-1 integrase inhibitor peptides greatly improved cell permeability and inhibited HIV-1 replication in cell cultures (Y-Q
et al, J. Med. Chem., Just Accepted Manuscript, Published on Web: June 12, 2013).
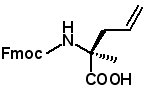
Fmoc-(2R)-2-(2-propenyl)alanine 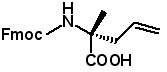
Fmoc-(2S)-2-(3-butenyl)alanine 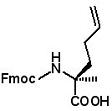
Fmoc-(2-R)-2-(3-butenyl)alanine 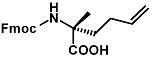
Fmoc-(2S)-2-(3-pentenyl)alanine 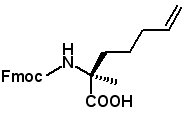
Fmoc-(2-R)-2-(4-pentenyl)alanine 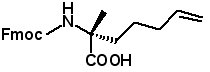
Fmoc-(2S)-2-(7-octenyl)alanine 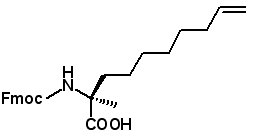
Fmoc-(2-R)-2(7-octenyl)alanine 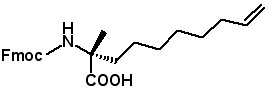
Fmoc-bis-(4-pentenyl)glycine
R. Bruce Merrifield, the Nobel prize winning chemist who invented solid phase peptide synthesis was born July 15, 1921. |
 |
UPCOMING EVENTS |
|
|
August 2013 15th International Congress of Immunology August 22-27 Milan, Italy September 2013 22nd Polish Peptide Symposium September 1-5 Peptide Arrays 2013 September 3-4 |
 |