|
 |
|
Featured Articles A convenient solid-phase strategy for the synthesis of antimicrobial cyclic lipopeptides. Vilà S, Badosa E, Montesinos E, Feliu L, Planas M., Org Biomol Chem. 2013, 11, 3365-74. A concise solid-phase synthesis of cyclic lipopeptides derived from the antimicrobial peptide c(Lys-Lys-Leu-Lys-Lys-Phe-Lys-Lys-Leu-Gln) () was accomplished. Three different synthetic routes were explored. Best results were obtained using a protocol that includes as key steps: (i) synthesis of the cyclic peptidyl resin incorporating the Lys residue to be acylated protected at the N(ε)-amino group with an ivDde group, (ii) selective removal of the ivDde group, and (iii) acylation. These compounds were screened for their in vitro growth inhibition of bacterial and fungal phytopathogens and for their cytotoxic effects on eukaryotic cells. A sequence with high antimicrobial activity and low hemolysis was identified, constituting a good candidate for the design of new antimicrobial agents. Synthesis of d-Abrines by Palladium-catalyzed Reaction of ortho-Iodoanilines with N-Boc-N-methylalanyl-substituted Acetaldehyde and Acetylene Paulami Danner , Marius Morkunas , and Martin E. Maier, Org. Lett., Article ASAP A novel strategy to N-Boc-N-methyl--tryptophans (abrine derivatives) was developed that relies on the palladium-catalyzed annulation of ortho-iodoanilines 12 with either N-Boc-N-methyl-propargylglycine 16 or aldehyde 11. Both 11 and 16 can be prepared from d-serine. An alternative route to propargylglycine 16 utilizes an enantioselective propargylation reaction of glycine imine 17. Citric acid-assisted two-step enrichment with TiO2 enhances the separation of multi- and mono-phosphorylated peptides and increases phosphoprotein profiling Xuyang Zhao , Qingsong Wang , Shuxin Wang , Xiao Zou , Mingrui An , Xuefei Zhang , and Jianguo Ji J. Proteome Res., Just Accepted Manuscript Phosphopeptide enrichment is essential for large-scale phosphoprotein profiling. Titanium dioxide (TiO2) is widely used in phosphopeptide enrichment, but is limited in the isolation of multi-phosphorylated peptides due to their strong binding. In this study, we found that citric acid greatly affects the binding of mono- and multi-phosphopeptides with TiO2, which can be used for step-wise phosphopeptide separation coupled with mass spectrum (MS) identification. We first loaded approximately 1 mg peptide mixture of HeLa cell digests onto TiO2 beads in highly concentrated citric acid (1 M). Then the flow-through fraction was diluted to ensure low concentration of citric acid (50 mM) and followed by loading onto another aliquot of TiO2 beads. The two eluted fractions were subjected to nanoLC-MS/MS analysis. We identified 1,500 phosphorylated peptides, of which 69% were multi-phosphorylated after the first enrichment. After the second enrichment, 2,167 phosphopeptides, of which 92% were mono-phosphorylated, were identified. In total, we successfully identified 3,136 unique phosphopeptides containing 3,973 phosphosites utilizing this strategy. Finally, more than 37% of the total phosphopeptides and 2.6-fold more of the multi-phosphorylated peptides were identified as compared to the frequently used DHB/TiO2 enrichment strategy. Combining SCX with CATSET, we identified 14,783 phosphopeptides and 15,713 phosphosites, of which 3,678 were unrecorded in PhosphoSitePlus database. This two-step separation procedure for sequentially enriching multi- and mono-phosphorylated peptides by using citric acid is advantageous in multi-phosphorylated peptide separation, as well as for more comprehensive phosphoprotein profiling. A large synthetic peptide and phosphopeptide reference library for mass spectrometry-based proteomics. L. Wei, J. Yang, X. He, G. Mo, J. Hong, X. Yan, D. Lin, R. Lai, J. Med. Chem., Just Accepted Manuscript DOI: 10.1021/jm4004158 Publication Date (Web): April 17, 2013 Copyright © 2013 American Chemical Society We present a peptide library and data resource of >100,000 synthetic, unmodified peptides and their phosphorylated counterparts with known sequences and phosphorylation sites. Analysis of the library by mass spectrometry yielded a data set that we used to evaluate the merits of different search engines (Mascot and Andromeda) and fragmentation methods (beam-type collision-induced dissociation (HCD) and electron transfer dissociation (ETD)) for peptide identification. We also compared the sensitivities and accuracies of phosphorylation-site localization tools (Mascot Delta Score, PTM score and phosphoRS), and we characterized the chromatographic behavior of peptides in the library. We found that HCD identified more peptides and phosphopeptides than did ETD, that phosphopeptides generally eluted later from reversed-phase columns and were easier to identify than unmodified peptides and that current computational tools for proteomics can still be substantially improved. These peptides and spectra will facilitate the development, evaluation and improvement of experimental and computational proteomic strategies, such as separation techniques and the prediction of retention times and fragmentation patterns. Understanding acid lability of cysteine protecting groups. Ramos-Tomillero I, Mendive-Tapia L, Góngora-Benítez M, Nicolás E, Tulla-Puche J, Albericio F. Cys-disulfide bonds contribute to the stabilization of peptide and protein structures. The synthesis of these molecules requires a proper protection of Cys residues, which is crucial to prevent side-reactions and also to achieve the correct Cys connectivity. Here we undertook a mechanistic study of a set of well-known acid-labile Cys protecting groups, as well other new promising groups, in order to better understand the nature of their acid-lability. The stability of the carbocation generated during the acid treatment was found to have a direct impact on the removal of the protective groups from the corresponding protected Cys-containing peptides. Hence a combination of steric and conjugative effects determines the stability of the carbocations generated. Here we propose diphenylmethyl (Dpm) as a promising protecting group on the basis of its intermediate relative carbocation stability. All the optimized geometries and energies presented in this study Urodilatin regulates renal dopamine metabolism. Choi MR, Citarella MR, Lee BM, Kouyoumdzian NM, Rukavina Mikusic NL, Fernández BE. Background: Sodium and water transport across renal proximal tubules is regulated by diverse hormones such as dopamine and urodilatin. We have previously reported that urodilatin stimulates extraneuronal dopamine uptake in external renal cortex by activation of the type A natriuretic peptide receptor, coupled to cyclic guanylate monophosphate signaling and protein kinase G. Moreover, urodilatin enhances dopamine-induced inhibition of Na+, K+-ATPase activity in renal tubules. The aim of the present study was to evaluate whether urodilatin could also alter renal dopamine synthesis, release, catabolism and turnover. |
|
Infinity 2400TM Slow or difficult deprotection and coupling reactions reduce yields and result in crude peptides containing truncation and deletion peptide impurities. AAPPTec’s Infinity 2400 Peptide Synthesizer utilizes microwaves for rapid heating that can accelerate slow reactions, improve yields and produce purer peptides of difficult sequences.
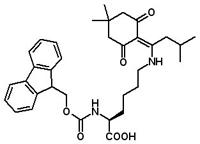
Fmoc-Lys(ivDde)-OH
Fmoc-Lys(ivDde)-OH is a useful building block for preparing peptides with selective modification on the lysine sidechain. Commonly, such modifications include labeling with a biotin tag, fluorescence labeling with dansyl of fluorescenyl, or cyclization at the lysine sidechain. ivDde protection of the lysine side chain was recently utilized in solid phase synthesis of cyclic lipopeptides (S. Vilà, et al. Org Biomol Chem. 2013, 11, 3365-74). The ivDde is resistant to TFA and piperidine, but can be removed with hydrazine.
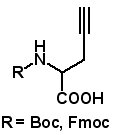
Propargylglycine
Propargyglycine derivatives were recently utilized to prepare tryptophan derivatives (P. Danner, et al. Org. Lett. Article ASAP, Published (Web): April 30, 2013). The indol ring was formed by palladium-catalyzed annulation of ortho-iodoanilines. Propargylglycine also undergoes Cu(I)-catalyzed cycloaddition with azides (click chemistry) in high yield. AAPPTec provides Fmoc and Boc protected propargylglycines. (For AAPPTec’s azido amino acids for click chemistry, click HERE).
Urodilatin Urodilatin was originally isolated from human urine. It belongs to the family of natriuretic-vasorelaxant peptides found in the heart atria. it was recently reported that urodilatin regulates renal dopamine metabolism. M. Gagelmann, et al., FEBS Lett., 1988, 233, 239; V. di Marzo, Trends Pharmacol. Sci., 1989, 10, 91; A.J. Kenny, et al., Biochem. J., 1993, 291, 83; C. Cedidi, et al., Eur. J. Clin. Invest., 1994, 24, 632;T. Flüge, et al., Eur. J. Pharmacol., 1994, 271, 395; M.R. Choi, et al., J Nephrol. Published on Web: May 6, 2013.
ACTEvap: Affordable Compact Tabletop Evaporator/Lyophylizer The ACTEvap is useful in any laboratory for:
AAPPTec Peptide Catalog AAPPTec’s On Line Peptide Catalog contains over 2100 peptide products at very competitive prices. The Peptide Catalog includes peptides in these classes: • Amyloids
|

|
UPCOMING EVENTS |
|
|
June 2013 23rd American Peptide Symposium June 22-27 Hilton Waikoloa Village, Hawaii
|
 |