|
 |
|
Recent Publications Antimicrobial peptides incorporating non-natural amino acids as agents for plant protection. Ng-Choi I, Soler M, Guell I, Badosa E, Cabrefiga J, Bardaji E, Montesinos E, Planas M, Feliu L., Protein Pept Lett., 2014, 21, 357-67. The control of plant pathogens is mainly based on copper compounds and antibiotics. However, the use of these compounds has some limitations. They have a high environmental impact and the use of antibiotics is not allowed in several countries. Moreover, resistance has been developed to these pathogens. The identification of new agents able to fight plant pathogenic bacteria and fungi will represent an alternative to currently used antibiotics or pesticides. Antimicrobial peptides are widely recognized as promising candidates, however naturally occurring sequences present drawbacks that limit their development. These include susceptibility to protease degradation and low bioavailability. To overcome these problems, research has focused on the introduction of unnatural amino acids into lead peptide sequences. In particular, we have improved the biological profile of antimicrobial peptides active against plant pathogenic bacteria and fungi by incorporating triazolyl, biaryl and D-amino acids into their sequence. These modifications and their influence on the biological activity are summarized. Hydroxyproline substitutions stabilize non-glycosylated drosocin against serum proteases without challenging its antibacterial activity. Knappe D, Cassone M, Nollmann FI, Otvos L, Hoffmann R., Protein Pept Lett., 2014, 21, 321-9. The increasing incidence of multi- and pan-resistant pathogens demands novel compounds to fight Grampositive and especially Gram-negative bacteria. Among the currently investigated compound classes antimicrobial peptides (AMPs) inhibiting specific bacterial targets appear especially promising for systemic therapy of infections, although unmodified linear peptides are typically rapidly degraded by serum proteases. Proline-rich AMPs have been heavily investigated in recent years due to their low toxicity and proven in vivo efficacy. Here, we report novel unglycosylated drosocin analogs with extended half-life in mouse serum and improved activity against Gram-negative pathogens Escherichia coli and Klebsiella pneumoniae. Substituting proline (Pro) residues in positions 3, 5, 10, and 14 with trans-4-hydroxy-L-proline ((t)Hyp) improved the antibacterial activity, whereas substitution of Pro-16 reduced the activity. Drosocin analogs with (t)Hyp in positions 3 and 5 were also four to eight times more stable in mouse serum than the unmodified analog. The new compounds were not toxic against human HeLa, HEK293, and HepG2 cell lines and showed no hemolytic activity against human erythrocytes at peptide concentrations of at least 600 μg/mL. Cysteine Pseudoprolines for Thiol Protection and Peptide Macrocyclization Enhancement in Fmoc-Based Solid-Phase Peptide Synthesis Postma TM, Albericio F., Org. Lett., Article ASAP DOI: 10.1021/ol5004725 Publication Date (Web): March 11, 2014 Copyright © 2014 American Chemical Society Contrary to other studies, here we describe cysteine (Cys) pseudoproline-containing peptides with short deprotection times in TFA. The deprotection times fell in the same range as other protecting groups commonly used in SPPS (e.g., 1-3 h). Moreover, when using Cys pseudoprolines as peptide macrocyclization-enhancing moieties a considerable reduction in reaction time was observed compared to a peptide containing trityl protected Cys. Cyclo(d-Tyr-d-Phe): a new antibacterial, anticancer, and antioxidant cyclic dipeptide from Bacillus sp. N strain associated with a rhabditid entomopathogenic nematode. Nishanth Kumar S, Dileep C, Mohandas C, Nambisan B, Ca J., J Pept Sci., 2014, 20, 173-85. A new microbial cyclic dipeptide (diketo-piperazine), cyclo(d-Tyr-d-Phe) was isolated for the first time from the ethyl acetate extract of fermented modified nutrient broth of Bacillus sp. N strain associated with rhabditid Entomopathogenic nematode. Antibacterial activity of the compound was determined by minimum inhibitory concentration and agar disc diffusion method against medically important bacteria and the compound recorded significant antibacterial against test bacteria. Highest activity was recorded against Staphylococcus epidermis (1μg/ml) followed by Proteus mirabilis (2μg/ml). The activity of cyclo(d-Tyr-d-Phe) against S. epidermis is better than chloramphenicol, the standard antibiotics. Cyclo(d-Tyr-d-Phe) recorded significant antitumor activity against A549 cells (IC50 value: 10μM) and this compound recorded no cytotoxicity against factor signaling normal fibroblast cells up to 100μM. Cyclo(d-Tyr-d-Phe) induced significant morphological changes and DNA fragmentation associated with apoptosis in A549 cells. Acridine orange/ethidium bromide stained cells indicated apoptosis induction by cyclo(d-Tyr-d-Phe). Flow cytometry analysis showed that the cyclo(d-Tyr-d-Phe) did not induce cell cycle arrest. Effector molecule of apoptosis such as caspase-3 was found activated in treated cells, suggesting apoptosis as the main mode of cell death. Antioxidant activity was evaluated by free radical scavenging and reducing power activity, and the compound recorded significant antioxidant activity. The free radical scavenging activity of cyclo(d-Tyr-d-Phe) is almost equal to that of butylated hydroxyanisole, the standard antioxidant agent. We also compared the biological activity of natural cyclo(d-Tyr-d-Phe) with synthetic cyclo(d-Tyr-d-Phe) and cyclo(l-Tyr-l-Phe). Natural and synthetic cyclo(d-Tyr-d-Phe) recorded similar pattern of activity. Although synthetic cyclo(l-Tyr-l-Phe) recorded lower activity. But in the case of reducing power activity, synthetic cyclo(l-Tyr-l-Phe) recorded significant activity than natural and synthetic cyclo(d-Tyr-d-Phe). The results of the present study reveals that cyclo(d-Tyr-d-Phe) is more bioactive than cyclo(l-Tyr-l-Phe). To the best of our knowledge, this is the first time that cyclo(d-Tyr-d-Phe) has been isolated from microbial natural source and also the antibacterial, anticancer,and antioxidant activity of cyclo(d-Tyr-d-Phe) is also reported for the first time. Copyright © 2013 European Peptide Society and John Wiley & Sons, Ltd. Immobilized N-Chlorosuccinimide as a Friendly Peptide Disulfide Forming Reagent Postma TM, Albericio F., ACS Comb. Sci., Just Accepted Manuscript DOI: 10.1021/co500003p Publication Date (Web): March 18, 2014 Copyright © 2014 American Chemical Society A novel immobilized N-chlorosuccinimide resin was developed for peptide disulfide bond formation in combinatorial libraries. The resin is prepared in a simple two-step process from commercial starting materials. Disulfide formation is initiated by adding a peptide solution to the resin, and excess reagent is removed by a convenient filtration upon completion of disulfide formation. Completion of disulfide formation is rapid and clean, as demonstrated by the oxidation of a small nonapeptide library. This immobilized reagent allows a wider scope for the use of N-chlorosuccinimide based disulfide formation in combinatorial chemistry. Unexpected Hydrolytic Instability of N-Acylated Amino Acid Amides and Peptides Samaritoni JG, Copes AT, Crews DeMK, Glos C, Thompson AL, Wilson C, O'Donnell MJ, Scott WL., J. Org. Chem., Just Accepted Manuscript DOI: 10.1021/jo500273f Publication Date (Web): March 12, 2014 Copyright © 2014 American Chemical Society Remote amide bonds in simple N-acyl amino acid amide or peptide derivatives 1 can be surprisingly unstable hydrolytically, affording in solution variable amounts of 3 under mild acidic conditions, such as trifluoroacetic acid/water mixtures at room temperature. This observation has important implications for the synthesis of this class of compounds, which includes N-terminal acylated peptides. We describe the factors contributing to this instability and how to predict and control it. The instability is a function of the remote acyl group R2CO four bonds away from the site of hydrolysis. Electron-rich acyl R2 groups accelerate this reaction. In the case of acyl groups derived from substituted aromatic carboxylic acids the acceleration is predictable from the substituent's Hammett sigma value. N-capped dipeptides are also hydrolyzed under typical cleavage conditions. This suggests that unwanted peptide truncation may occur during synthesis or prolonged standing in solution when dipeptides or longer peptides are acylated on the N-terminus with electron-rich aromatic groups. When amide hydrolysis is an undesired secondary reaction, as can be the case in the trifluoroacetic acid-catalyzed cleavage of amino acid amide or peptide derivatives 1 from solid-phase resins, conditions are provided to minimize that hydrolysis. |
|
Unusual Amino Acids Unusual amino acids are essential building blocks for probing receptor structure and developing high potency, highly selective agonist and antagonists. AAPPTec offers a large library of unusual amino acids for peptide synthesis. Beta-amino acids, substituted phenylalanines, homo-amino aicds, beta-homo amino acids, alkenyl amino acids, azido amino acids, hydroxyprolines, and amino acid analogs are some of the many types of unusual amino acids in AAPPTec's catalog. L-isomers, D-isomers, Boc-protected, Fmoc-protected and N-unprotected derivatives are available. Please visit our website www.aapptec.com for our full catalog of unusual amino acid products. PEG Linkers Unlike hydrocarbon linkers that are hydrophobic, PEG linkers are hydrophilic and help improve the solubility characteristics of hydrophobic peptides or peptides labeled with hydrophobic tags. AAPPTec PEG linkers have uniform established structures and sizes, allowing you to incorporate them and obtain well-defined products.
For additional PEG products from AAPPTec, go to the PEGylation Reagents page of our website www.aapptec.com. Over 2000 catalog peptides available online at www.aapptec.com.
Apex 396 
The Apex 396 is the premier productivity tool. Capable of preparing a few peptides or up to 96 separate peptides at the same time, the Apex 396 is ideal for SAR studies, optimization studies, drug discovery, and small scale peptide production.
Fluorescent Dyes
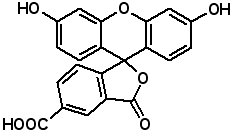
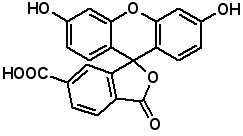
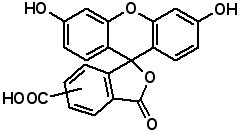
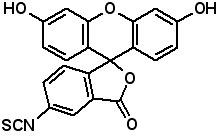
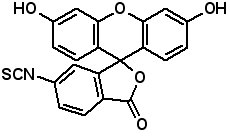
Fluorescence labeled peptides are used to study enzyme kinetics, transport of peptides into cells and the disposition of peptides within cells, tissues and organs. AAPPTec offers five fluorescein-based reagents for preparing labeled peptides, 5-FAM, 6-FAM, 5(6)-FAM, 5-FITC and 6-FITC. These reagents react with amines to form labeled compounds that fluoresce green when illuminated with UV light. Procedures for on-resin peptide labeling with these reagents can be found on our website www.aapptec.com in the Resources section under Protocols.
,. 
Titan 357 Combinatorial Library Synthesizer
The Titan 357 Combinatorial Peptide Library Synthesizer is the most efficient and easiest-to-use instrument for preparing one-bead-one-compound combinatorial libraries. Combinatorial
peptide libraries provide the means for preparing and screening very large numbers of peptides.
|
 |
UPCOMING EVENTS |
|
|
June 2014 3rd International Symposium on Microwave and Alternative Organic and Peptide Synthesis June 25-26 |
 |