|
 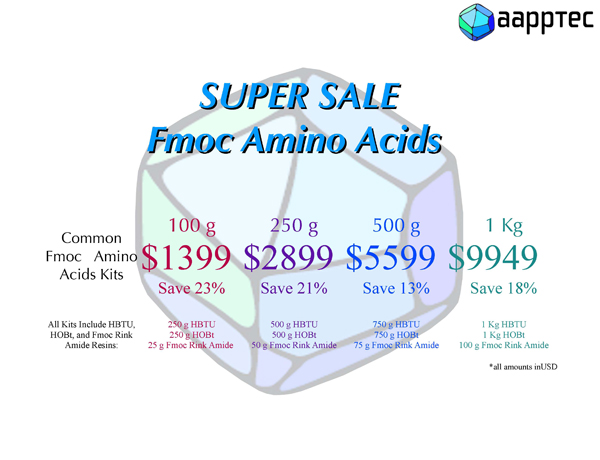 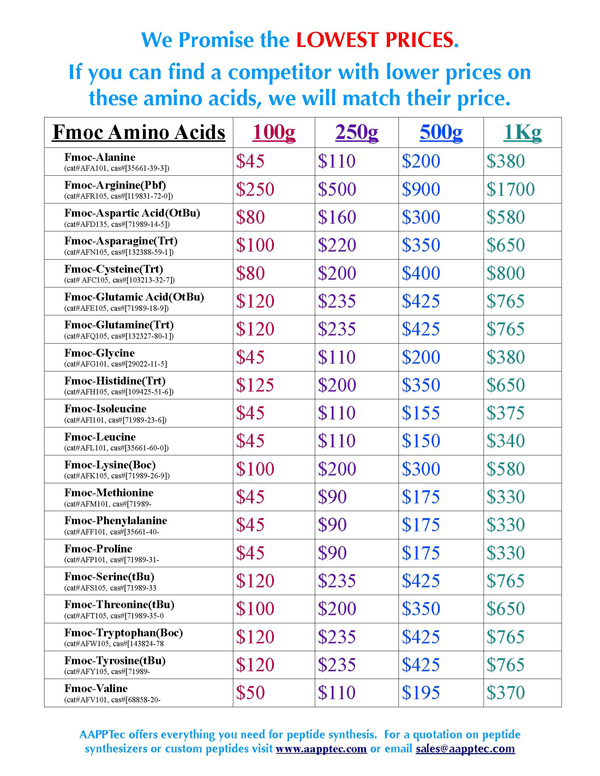 |
|
News New Antifungal Peptides Invasive fungal infections such as invasive aspergillosis and invasive candidiasis are associated with high mortality. The fungus Candida is the most common cause of heathcare-associated bloodstream infections in the United States. (SS Magill, et al., NewEngland J. Med., 2014, 370, 1198-208) Some types of Candida are becoming increasingly resistant to common antifungal medications. Resistance in Aspergillis is an emerging issue, too.
New Application of the Tetrahydropyranyl Protecting Group Fernando Albericio has reported utilizing the tetrahydropyranyl (Thp) group as an acid labile Cys protecting group for Fmoc peptide chemistry. (I Ramos-Tomillero, et al., Org.Lett., 2015, 17, 1680-8) It was shown to be superior to Trt, Dpm, Acm and StBu in solid phase peptide synthesis. Cys racemization and C-terminal 3-(1-piperdinyl)alanine formation were minimized with Cys(Thp).
Trp-Trp Cross-Linking: A Structure-Reactivity Relationship in the Formation and Design of Hyperstable Peptide β-Hairpin and α-Helix Scaffolds KM Makwana and R Mahalakshmi, Org. Lett., Article ASAP, DOI: 10.1021/acs.orglett.5b01017 Publication Date (Web): May 4, 2015 Copyright © 2015 American Chemical Society Using model peptide β-hairpin scaffolds, the facile formation of a remarkably stable covalently cross-linked modification is reported in the tryptophan side chain, which confers hyperstability to the scaffold and displays a unique structure–reactivity relationship. This strategy is also validated to obtain a thermostable α-helix. Such imposition of conformational constraints can have versatile applications in peptide-based drug discovery, and this strategy may improve peptide bioavailability. Catalytic Chemical Amide Synthesis at Room Temperature: One More Step Toward Peptide Synthesis T Mohy E Dine, W Erb, Y Berhault, J Rouden, J Blanchet, J. Org. Chem., 2015, 80, 4532-44. Copyright © 2015 American Chemical Society An efficient method has been developed for direct amide bond synthesis between carboxylic acids and amines via (2-(thiophen-2-ylmethyl) phenyl)boronic acid as a highly active bench-stable catalyst. This catalyst was found to be very effective at room temperature for a large range of substrates with slightly higher temperatures required for challenging ones. This methodology can be applied to aliphatic, a-hydroxyl, aromatic, and heteroaromatic acids as well as primary, secondary, heterocyclic, and even functionalized amines. Notably, N-Boc-protected amino acids were successfully coupled in good yields with very little racemization. An example of catalytic dipeptide synthesis is reported. Self-Assembly of a Nine-Residue Amyloid-Forming Peptide Fragment of SARS Corona Virus E-Protein: Mechanism of Self Aggregation and Amyloid-Inhibition of hIAPP A Ghosh, AS Pithadia, J Bhat, S Bera, A Midya, CA Fierke, A Ramamoorthy, A Bhunia, Biochemistry, 2015, 54, 2249-61. Copyright © 2015 American Chemical Society Molecular self-assembly, a phenomenon widely observed in nature, has been exploited through organic molecules, proteins, DNA, and peptides to study complex biological systems. These self-assembly systems may also be used in understanding the molecular and structural biology which can inspire the design and synthesis of increasingly complex biomaterials. Specifically, use of these building blocks to investigate protein folding and misfolding has been of particular value since it can provide tremendous insights into peptide aggregation related to a variety of protein misfolding diseases, or amyloid diseases (e.g., Alzheimer’s disease, Parkinson’s disease, type-II diabetes). Herein, the self-assembly of TK9, a nine-residue peptide of the extra membrane C-terminal tail of the SARS corona virus envelope, and its variants were characterized through biophysical, spectroscopic, and simulated studies, and it was confirmed that the structure of these peptides influences their aggregation propensity, hence, mimicking amyloid proteins. TK9, which forms a beta-sheet rich fibril, contains a key sequence motif that may be critical for beta-sheet formation, thus making it an interesting system to study amyloid fibrillation. TK9 aggregates were further examined through simulations to evaluate the possible intra- and interpeptide interactions at the molecular level. These self-assembly peptides can also serve as amyloid inhibitors through hydrophobic and electrophilic recognition interactions. Our results show that TK9 inhibits the fibrillation of hIAPP, a 37 amino acid peptide implicated in the pathology of type-II diabetes. Thus, biophysical and NMR experimental results have revealed a molecular level understanding of peptide folding events, as well as the inhibition of amyloid-protein aggregation are reported. A peptide inhibitor derived from the conserved ectodomain region of DENV membrane (M) protein with activity against Dengue virus infection. A Panya, N Sawasdee, M Junking, C Srisawat, K Choowongkomon, PT Yenchitsomanus, Chem Biol Drug Des., 2015 Apr 17. Accepted Article doi: 10.1111/cbdd.12576. [Epub ahead of print] Dengue virus (DENV) infection is a public health problem worldwide, thus the development of a vaccine and anti-DENV drugs are urgently needed. It has been observed that low levels of viremia in DENV-infected individuals are associated with mild disease outcomes; therefore, reduction of DENV load should offer therapeutic benefits. Disruption of protein-protein interactions on the surface of DENV by a peptide that mimics part of its structural protein may affect stability of the virion structure and inhibit viral entry into host cells. To test this hypothesis, we generated a novel peptide inhibitor that mimics the conserved ectodomain region of DENV membrane (M) protein, MLH40 peptide, for DENV inhibition assays. MLH40 inhibited all four serotypes of the virus (DENV1-4) at half maximal inhibition concentration of 24-31 µM. MLH40 at 100 µM blocked DENV2 attachment to cells by 80%. The inhibitory activity of MLH40 against DENV was consistently observed with different cell types, including Vero, A549, and Huh7 cells. Prediction of MLH40 binding by a molecular docking program indicated its N-terminal loop may interact with DENV envelope (E) proteins and alter their dimer conformation. Thus, MLH40 may serve as a lead-peptide inhibitor for the development of an anti-DENV drug. This article is protected by copyright. All rights reserved. Peptide Macrocycles Featuring a Backbone Secondary Amine: A Convenient Strategy for the Synthesis of Lipidated Cyclic and Bicyclic Peptides on Solid Support A Oddo, L Münzker, PR Hansen, Org. Lett. Article ASAP, Publication Date (Web): April 29, 2015 DOI: 10.1021/acs.orglett.5b01026 Copyright © 2015 American Chemical Society A convenient strategy for the on-resin synthesis of macrocyclic peptides (3- to 13-mers) via intramolecular halide substitution by a diamino acid is described. The method is compatible with standard Fmoc/tBu SPPS and affords a tail-to-side-chain macrocyclic peptide featuring an endocyclic secondary amine. This functional group is still reactive toward acylation, allowing for the continuation of the synthesis. An application to the synthesis of lipidated cyclic and bicyclic antimicrobial peptides is presented. Design of a Potent Antibiotic Peptide Based on the Active Region of Human Defensin 5 C Wang, M Shen, N Gohain, WD Tolbert, F Chen, N Zhang, K Yang, A Wang, Y Su, T Cheng, J Zhao, M Pazgier, J Wang, J. Med. Chem., 2015, 58, 3083-93. Copyright © 2015 American Chemical Society Human defensin 5 (HD5) is a broad-spectrum antibacterial peptide with a C-terminal active region. To promote the development of this peptide into an antibiotic, we initially substituted Glu21 with Arg because it is an electronegative residue located around the active region. Although detrimental to dimer formation, the E21R substitution markedly enhanced the antibacterial activity of HD5 and increased its ability to penetrate cell membranes, demonstrating that increasing the electropositive charge compensated for the effect of dimer disruption. Subsequently, a partial Arg scanning mutagenesis was performed, and Thr7 was selected for replacement with Arg to further strengthen the antibacterial activity. The newly designed peptide, T7E21R-HD5, exhibited potent antibacterial activity, even in saline and serum solutions. In contrast to monomeric E21R-HD5, T7E21R-HD5 assembled into an atypical dimer with parallel ß strands, thus expanding the role of increasing electropositive charge in bactericidal activity and providing a useful guide for further defensin-derived antibiotic design.
|
|
Lipidized Peptides Attaching lipid groups to peptides often improves their drug-like characteristics. Lipidization of prolactin-releasing peptide analogs enhanced their stability and mediated their effect in the central nervous system. (L Maletínská, et al., Int. J. Obes., 2015, Mar 16. DOI: 10.1038/ijo.2015.28) Adding a myristoyl tag to a CRMP2 peptide aptamer enhanced its intracellular delivery and increased local concentration of the peptide aptamer near calcium channels, resulting in better anti-nociceptive potential. (L François-Moutal, et al., Pain, 2015, Mar 6. DOI: 10.1097/j.pain.0000000000000147) Adding lipid groups to diapause hormone analogs produced products that penetrate pupae of Heilicoverpa zea when applied topically and terminate pupal diapause. (Q Zhang, et al., Insect Mol. Biol., 2015, Mar 6 DOI: 10.1038/ijo.2015.28)
AAPPTec Products in the Literature
This is an example of how AAPPTec’s peptide reagents and synthesizers are being used in current research.
A Lanthanide(III) Triflate Mediated Macrolactonization/Solid-Phase Synthesis Approach for Depsipeptide Synthesis
JD Goodreid, E da Silveira dos Santos, RA Batey, Org. Lett., 2015, 17, 2182-5.
Copyright © 2015 American Chemical Society
Focus XC-6 The Focus XC-6 can prepare up to six different peptides at the same time on your benchtop. This compact instrument offers the features and flexibility of larger instruments but in a smaller footprint. The Focus XC features six solvent and reagent bottles, 24 amino acid/reagent vessels and a preactivation - measuring vessel for preactivation and precise delivery of amino acids.
Anticonvulsant Activity of Neuropeptide FF and Prolactin-Releasing Peptide Intracerebroventricular infusion of neuropeptide FF or prolactin-releasing peptide decreases cortical excitability in rats. (I Buffel, et al., Epilepsia, 2015, 56, 489-98) These peptides are potential leads for the development of new drugs to treat epilepsy. Neuropeptide FF and Prolactin-Releasing Peptide are available from AAPPTec. Cysteine Derivatives for SPPS  Peptides containing multiple disulfide bridges, such as human insulin or a-conotoxin GS, require special planning and cysteine derivatives with side chain protecting groups that can be removed selectively. A recent publication in Organic Letters describes the regioselective formation of four disulphide bonds in a synthesis of human hepcidin. (M Mochizuki, et al., Org. Lett., 2015, 17, 2202-5) AAPPTec offers a variety of high purity Fmoc-Cys derivates for solid phase peptide synthesis.
Neuropeptide Y Neuropeptide Y is an important peptide in the brain. Activation of neuropeptide Y receptors in the bed nucleus of the stria terminalis suppresses binge alcohol drinking. (KE Pleil, et al., Nature Neuroscience, 2015, 18, 545-52) In the hypothalamus, neuropeptide Y stimulates autophagy. Since autophagy imparement contributes to age-related disease aggravation, modulating levels of neuropeptide Y in the hypothalamus may be a potential strategy to produce protective effects against hypothalamic impairments and to delay aging.
AAPPTec offers synthetic neuropeptide Y and neuropeptide Y fragments. Selective Melanocortin-4 Receptor Agonists and Antagonists Melanocortin-3 and -4 receptors in the brain play key roles in regulating feeding behaivior, metabolism and energy homostasis. Selective agonists and antagonists may lead to the development of new drugs to treat obesity and anorexia. A recently reported mouse melanocortin-4 receptor containing a ß3 homotryptophan residue showed 35-fold selectivity versus melanocortin-3 receptor. (A Singh, et al, ACS Med. Lett., Just Accepted Manuscript, Published ahead of print April 8, 2015, DOI: 10.1021/acsmedchemlett.5b00056) A ß-hairpin octapeptide mimetic of agouti-related protein (87-132) was also recently discovered which possesses sub-nanomolar antagonist activity at mouse melanocortin-4 receptors and greater than 160-fold selectivity versus mouse melanocortin-3 receptors. (MD Ericson, et al., J. Med. Chem., Just Accepted Manuscript, Published ahead of print April 21, 2015, DOI: 10.1021/acs.jmedchem.5b00184) Antimicrobial Peptide Derived from Centipedes Shows Anticancer Activity Scolopendrasin VII is an antimicrobial peptide derived from the Chinese red-headed centipede Scolopendra subspinipes mutilans. It was recently reported to induce necrosis in leukemia cells by specific interaction with phosphatidylserine. Phosphatidylserine is enriched in the membranes of cancer cells. (JH Lee, et al., J Microbiol. Biotechnol., Published online April 23, 2015, DOI: 10.4014/jmb.1503.03091) This may lead to an efficient new strategy for developing new anticancer drugs. |
 |
UPCOMING EVENTS |
|
June 2015 24th American Peptide Symposium June 20-25 June 26-30 July 2015 40th FEBS Congress July 4-9
|
 |

